Custom Sound Pro global usability strategy
Building a scalable, international usability testing framework for regulatory compliance and market expansion.

Building a scalable, international usability testing framework for regulatory compliance and market expansion.

Cochlear's flagship professional software hadn't seen a major update in 20 years. As we redesigned this critical tool used by audiologists worldwide, we faced a complex usability testing challenge.
Create a testing strategy that would not only meet regulatory compliance, but also scale our research capabilities globally whilst building local expertise across a specialised, distributed network of professionals. Equally important was the opportunity to strengthen relationships between HQ and regional teams by making them active partners in the product development process rather than passive recipients of centrally-designed solutions.
I was the senior UX designer responsible for the professional suite of products, but I was the hands-on, end-to-end designer for CS Pro. I was responsible for everything from research to wireframes, prototyping and visual design. In this case study I'm just talking about he usability.
Before implementing any testing protocols, we needed to build trust and demonstrate local relevance across diverse regional markets.
By involving regional stakeholders early and explaining how this means they can better serve the clinical accounts in their region, we built strong buy-in and enthusiasm for the project, before asking for participation.
Aside from regional buy-in, the biggest operational challenge was transforming non-UX professionals into confident, quality facilitators whilst managing technical constraints of secure clinical environments.
Many clinics operated on isolated networks for security, preventing online prototypes or real-time data collection from usability sessions.

Transformed non-UX professionals into confident, quality facilitators capable of collecting regulatory-standard data.
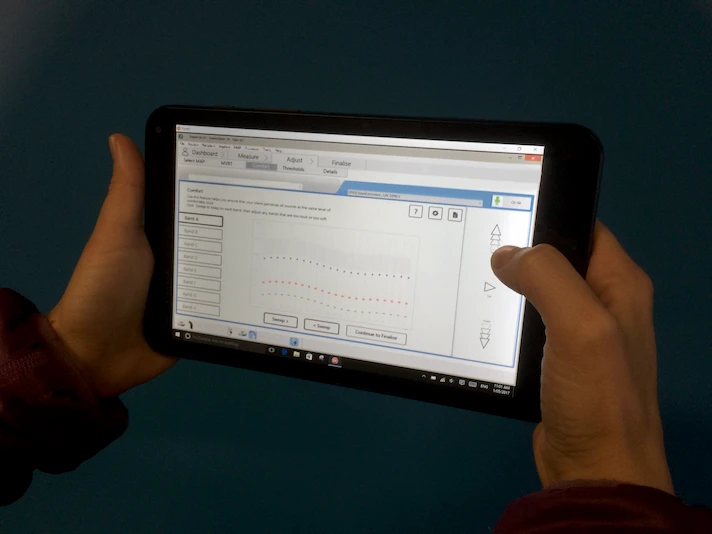
Balanced rapid iteration with thorough regulatory validation across diverse markets.
Created comprehensive evidence base meeting medical device regulatory standards whilst seamlessly integrating compliance into development workflows.
Usability sessions were facilitated in:
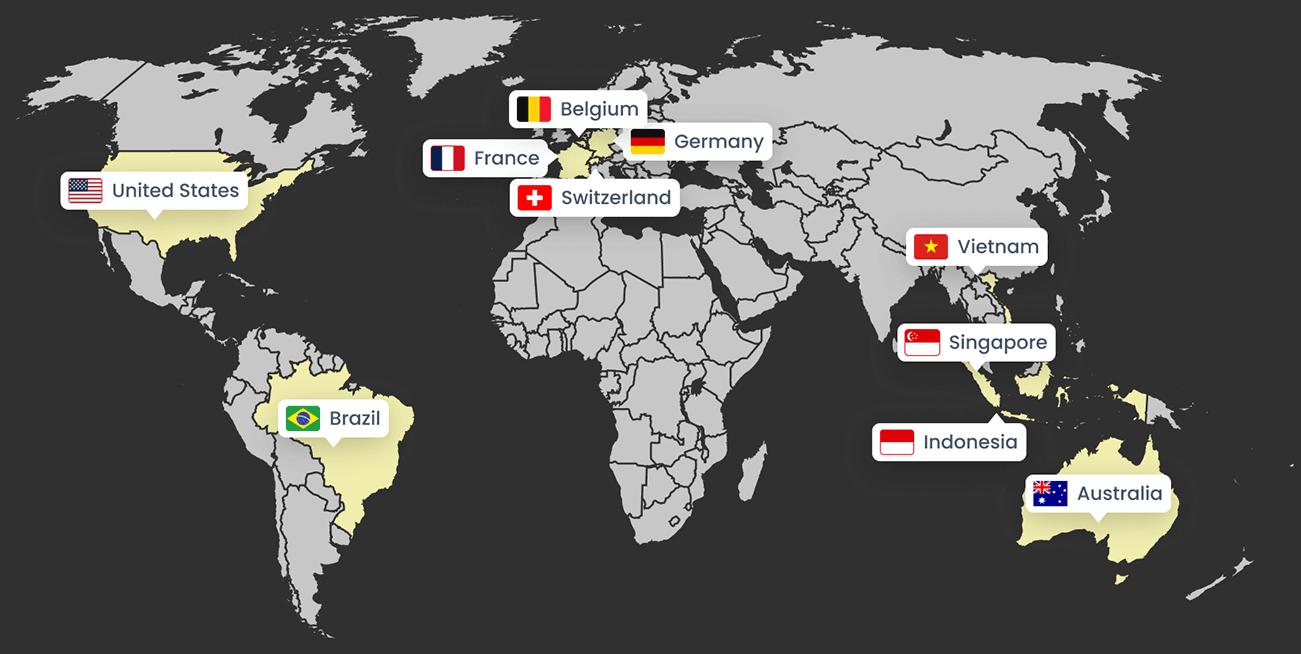
This global usability strategy supported the successful launch of Custom Sound Pro, now used by audiologists across thousands of clinics worldwide to improve hearing outcomes for the 1 million+ Cochlear implant recipients.
When building global research capabilities, constraints resulted in a more secure solution. Our offline prototype and our facilitator and observer questionnaires were more secure than our original online-first plan.
Regional buy-in required demonstrating local value, not just global benefits. The "Cochlear money" activity and persona validation created ownership rather than compliance.
In regulated industries, usability testing isn't just user advocacy, it's a systematic quality process where every finding must be actionable, traceable, and documented for compliance.
Global research requires balancing standardised processes with local flexibility. Success came from rigid data standards combined with local facilitation.
Creating a comprehensive digital accessibility resource for NSW Government
View case study